In recent years, cold chain logistics has become an increasingly complex process, thanks to stringent regulatory requirements and rapidly evolving demand in the food, medical and pharmaceutical sectors.
Increased disposable income has led to a worldwide increase in demand for fresh fruits, fish and meat and a preference for farm-to-table rather than frozen.
In the Pharmaceuticals/ Medical sector, a movement towards structurally complex, biotechnology and specialty formulations, as well as complex clinical trials has raised the stakes exponentially for cold chain logistics.
So how does a logistics provider grow and thrive in this challenging environment? Let us examine some best practices across the supply chain:
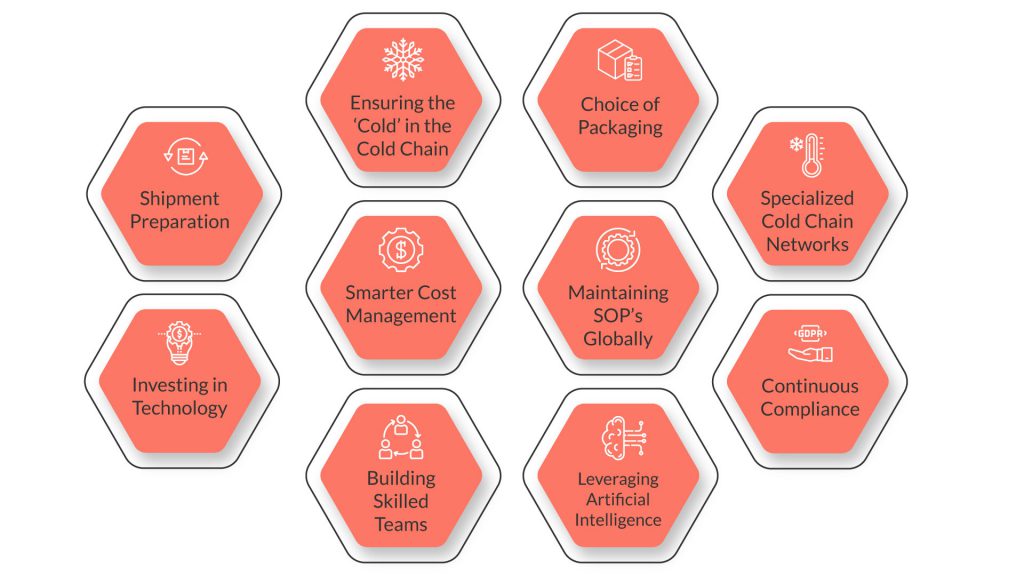
1.Shipment Preparation
Before anything else, it is important to understand the chemical/biological characteristics of the product, ideal temperature range and packaging requirements.
Most cold chain equipment is designed to maintain temperature at a specified level, not to bring the shipment to the required temperature. Hence cooling systems needs to be employed.
The demand for fresh produce is conditional- a single slip could affect the freshness and impact demand. Hence along with temperature, checking humidity and hygiene is also critical.
Transporting equipment such as reefers need to be pre cooled and steam cleaned in advance to avoid any chances of spoilage or contamination.
2.Ensuring the ‘Cold’ in the Cold Chain
Today, Cold chain technology offers temperature standards that can cater to a wide variety of products – from cryogenic (-135°C) to deep frozen, frozen, chilled and various controlled room temperatures (CRTs). However, staying within the required range at each stage of the cold chain is vital.
The ambient temperature during the journey and at the destination, as well as weather predictions needs to be considered. A reefer, with its own power supply can effectively handle these challenges.
It is also a versatile unit that can carry 20-25 tons of temperature sensitive cargo; hence it can be effectively used as an inter-modal transport system for global markets.
To prevent or control the ripening of fruits in-transit, atmospheric control is imperative. This can be achieved by wrapping the shipments in polyethylene bags, which will prevent gas from permeating.
Minimizing handling as well as unloading time is also the key to maintaining the integrity of the shipment.
Lastly, source loading can be a game changer. E.g., when it comes to meat exports, chilled rather than frozen meat is desirable. Freezing the meat may be easier for transportation, but actually lowers its quality.
However, if the meat is cut, inspected, vacuum sealed, boxed, palletized and loaded in a single chilled environment, the shelf life is increased substantially, and exporting chilled meat becomes viable.
3.Choice of Packaging
Packaging can be active or passive in terms of its approach and efficacy. Active packaging involves an energy source and thermostatic control (e.g., in the case of a reefer), plus monitoring mechanisms such as GPS and telematics.
Passive packaging involves conventional packages that use water/ ice/ dry ice to keep the product at required temperature. These shipments may be vulnerable to changes in ambient temperature, and to delays and disruptions in transportation.
However, the choice between the two modes must always consider the costs versus the benefits and risks. It must answer the question – how much risk can we take on?
Apart from the product value, risks also depends on regulatory compliance requirements, and possible loss of market share or brand value, should the product integrity be compromised.
4.Specialized Cold Chain Networks
In the case of temperature sensitive pharmaceutical products, all equipment and facilities at the logistics end must be highly specialized to handle these products, and actually serve as an extended arm of the manufacturer.
E.g., DHL has a Life Sciences and Healthcare division, which maintains certified stations near major airports, that operate on global GDP (Good Distribution Practices) standards. This ensures that shipments are held, handled and forwarded under controlled conditions.
Even warehouses that hold these shipments for an extended period are GDP certified, and provide value added services such as repacking, country specific labeling, end-to-end tracking, and compliance documentation.
5.Maintaining SOP’s Globally
Transporting high value goods globally through the cold chain requires well thought out policies and procedures.
The foundation for this is an assessment of in-transit / storage requirements and risk factors across the supply chain.
This could include what to do if there is a flight delay or if a container gets ruptured and product starts leaking etc. The SOP’s need to be standardized across markets, and the logistics provider needs to collaborate with the client on these SOP’s. The higher the risks involved, the tighter the collaboration needs to be.
6.Smarter Cost Management
While transporting pharmaceuticals, rather than managing costs on a purchase price basis, it is smarter to look at TCO (Total cost of Ownership).
This incorporates direct as well indirect costs such as risk of penalties, patient safety, loss of business/ market share/ brand value, investor sentiment etc. The hidden costs are the real risks facing the business, should the cold chain fail.
Hence, 3PLs need to realize that when they economize on one aspect of the cold chain without considering TCO, they put their client’s business at risk.
7.Investing in Technology
In the US, nearly a third of all food produced ends up being wasted. Out of this, about 12% occurs during the distribution process. To avoid spoilage, it is absolutely critical to ensure that food stuff is maintained at a specified temperature during transportation.
The only way to ensure that is to monitor temperature in real time. The best fleet management system can harness the power of GPS and telematics to monitor temperature, refer settings and humidity levels, along with position, battery level, door open/shut, risk of damage from shock etc.
It can send out updates and alert notifications in real time, so that the impact of breakdowns, power outages, open doors etc. is corrected before it starts to impact the product integrity.
8.Building Skilled Teams
Physical assets and technology can create a cold chain, but it is people with right skills that actually make it run. Be it food products or pharmaceuticals, logistics partners need to build teams that have knowledge and experience on the respective categories – product needs, handling of shipments and maintaining SOP’s.
Hence, creating niche teams and training them is a top priority. As a case in point, over 3000 DHL Life Sciences and Healthcare employees got trained in GDP.
9.Continuous Compliance
With the introduction of the Food Safety Modernization Act (FSMA), the FDA has started to take a proactive approach towards food safety, and preventing food contamination.
To meet this challenge, 3PL’s also need to be compliance experts – they need to monitor and log temperature and humidity for every shipment as it moves through the supply chain, and be able to furnish documentation as required.
Not only does this provide proof of product integrity to regulatory authorities, it also builds the confidence of customers. Also, in the event something does go wrong, these logs can be investigated to get to the source of the problem.
10.Leveraging Artificial Intelligence
The power of Big Data can be leveraged to better manage the cold chain. By working with data generated across thousands of shipments, it is possible to identify risk trends and devise ways to mitigate those risks.
Also, by looking at packaging performance across products, markets, temperature ranges, routes, transport modes etc. it could be possible to evaluate how a given packaging is likely to perform under a given set of parameters. This would help manufacturers shorten the qualification process for packaging.
Conclusion
The times are changing fast – as the global markets open up for food and pharmaceutical products, distribution processes and regulatory requirements will get more and more complex.
An intelligent fleet management software can help logistics partners ensure and provide proof that products are not only stored at the given temperature, but also kept within an approved range during every stage in the supply chain. After all, when it comes to cold chain logistics management, can you really afford to have any weak links?